Therapeutic peptides will be the most talked-about medical treatment in the coming decade, IF they are still around for the next 10 years from a legal and clinical perspective.
Especially since they have several advantages over other forms of medication:
“Therapeutic peptides have several important advantages over proteins or antibodies: they are small in size, easy to synthesise and have the ability to penetrate the cell membranes.
They also have high activity, specificity and affinity; minimal drug-drug interaction; and biological and chemical diversity.
An added benefit of using peptides as a treatment is that they do not accumulate in specific organs (e.g. kidney or liver), which can help to minimise their toxic side effects.
They can also be rapidly synthesized and easily modified and are less immunogenic than recombinant antibodies or proteins.”
(FYI: Peptides are categorized as having a chain of 40 amino acids as less, whereas anything more is considered as a protein/antibody)
And since they show great promise in treating a never-ending spectrum of diseases, there is no reason to NOT promote them relentlessly.
But there are some higher powers who have a vested interest in ensuring they never see the day of light.
And if they do, they will be for treating conditions far beyond the scope of their true value.
This sounds like an extraordinary claim if you take it at face value.
Which is why I’m going to take my time and slowly explain how peptides are fading away from clinical use.
Table of Contents
ToggleWhy I’m Focused On Preserving The Peptides Industry
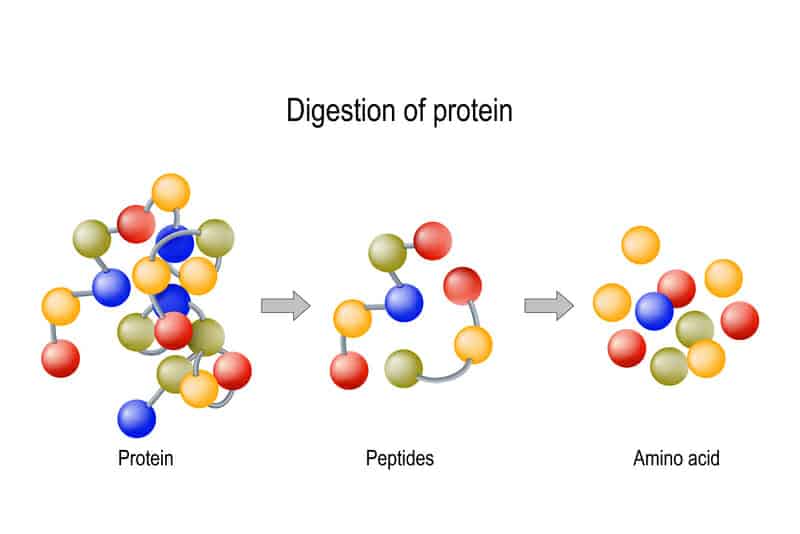
So here’s what has happened in recent times…
One of the world’s most prestigious peptide compounding pharmacies has been put on notice.
They received a ‘warning letter from the Federal Drug Administration (FDA)’ stating that some of the peptides they’ve been prescribing are being taken off the market as they are not FDA-approved:
That’s right – they’ve been synthesizing massively life-enhancing therapeutic peptides for the past 3 years, but no more.
This compounding pharmacy will have to stop producing these select peptides by a certain date to comply with the FDA mandate.
Why would the FDA do this?
If their primary mission is to protect consumers, globally and in the USA, why would they stop the distribution of proven and powerful life-altering medications that can save human lives?
To answer this question, we need to do some deep digging into several topics…
What Are Compounding Pharmacies And Why Do They Exist?

In case you have never heard of a compounding pharmacy, here is a brief description of what they do:
“Compounding is the creation of a pharmaceutical preparation—a drug—by a licensed pharmacist to meet the unique needs of an individual patient (either human or animal) when a commercially available drug does not meet those needs.
A patient may not be able to tolerate the commercially available drug, the exact preparation needed may not be commercially available, or a patient may require a drug that is currently in shortage or discontinued.”
Historically, there used to be a time in history where all drugs were compounded.
You would visit your local apothecary and the pharmacist would make it for you.
Doing so would allow the pharmacist to customize your formulation in a way that best suits you:
- Adding flavors to make a drug more digestible (think of a child who hates taking medication)
- Increasing or reducing the strength/dosage
- Removing certain allergens you may be sensitive to
- Altering the medium of the medication (taking a liquid instead of a pill, for instance)
Up until the 1900s, this was the very essence of the pharmacy field.
All of this changed when the Industrial Revolution came about and led to the mass manufacturing and production of standardized formulations.
So if a prescribed drug came in a dosage strength that was too high/low for you, or it had an additive you were allergic to, you were out of luck.
However, there still remained people who had specific and individualized medical needs.
For this reason, compounding pharmacies still existed but were largely left to the wayside.
It is only in very recent times where compounding pharmacies have begun to emerge again:
Funny how history ends up coming full circle to what our much wiser ancestors already know.
Unfortunately, it appears as if most of these compounding pharmacies may not be around for too much longer.
How Are Compounding Pharmacies Being Targeted?
To really get a clear picture of why compounding pharmacies are in jeopardy right now, it is important to understand the two types that exist:
503A Compounding Pharmacies
- “those that compound according to prescriptions specific to particular patients and are required by state boards of pharmacy to comply with USP and other guidelines”
- “limited to dispensing only for home use and are not allowed to compound large batches, an ability that can lead to lower product costs.”
503B Compounding Pharmacies
- “ those with outsourcing facilities that may manufacture large batches with or without prescriptions to be sold to healthcare facilities for office use only.”
- “allowed to use larger batches to lower their manufacturing costs, passing the savings onto consumers”
- “held to higher regulatory standards…These facilities are required to maintain full compliance with current good manufacturing practices (CGMP).”
As you can deduce from reading the descriptions above, the 503B pharmacies are the ones who would be synthesizing “grey market” peptides.
(FYI – “grey market” refers to a market in which products are distributed through unauthorized channels that do not belong to the original manufacturer)
If a doctor writes a script for a research chemical and then has a compounding pharmacy make it for the patient (assuming it was done under good judgment), the rules are technically being followed.
Now, this is where things get a little tricky.
When you only have a few 503B pharmacies that are operating under the radar without making too much noise, you won’t see the FDA hammering down on them quickly.
As the old saying goes, “We’ve got bigger fish to fry.”
But when too many people become aware of something that could disrupt the establishment, it’s going to get shut down in the blink of an eye.
For instance, if people purposely 10x their dose of a peptide thinking that 10x dose is automatically better, and then the consequent side effects gain enough volume, the FDA is going to take swift action and restrict access to said peptide.
In a weird way, the majority of the human population realistically needs the “mommy/daddy” FDA figure to tell them what to do and how to do it.
So now we have the 30,000-foot view of what happens, but let’s dive in deeper.
How Does The FDA Drug Approval Process Work?
Understanding the onslaught against therapeutic peptides requires you to know how the FDA approves drugs to be marketed and prescribed.
Here is an infographic taken directly from the FDA website that summarizes the process.


This picture also does a great job of showing just how notoriously difficult it is for a single drug to successfully pass through FDA’s drug approval process.
When you allow drugs that don’t go through the FDA process to mix into the public sphere (without their rigorous standard of testing), you compromise the entire system.
The FDA holds a position that until a particular drug product has been vetted to be safe and effective in their eyes, it should not be given to people.
To be clear, this is separate from prescribing drugs for off-label conditions:
“Off-label” drug use commonly refers to prescribing currently available medication for an indication (disease or symptom) for which it has not received FDA approval.
Off-label use also includes prescribing a drug for a different population or age range than that in which it was clinically tested and using a different dosage or dosage form.
Contrary to what patients might assume, off-label drug use is not the same as experimental or research use. Once a drug is FDA-approved for a specific indication, legally it can be used for any indication.
Off-label prescribing is common; it accounts for 10 to 20 percent of all prescriptions written, although the practice is more common in specific patient populations like children and the elderly.”
Here’s what all of this means:
The ONLY way a doctor can legally (and I do mean 100% legally) prescribe a therapeutic peptide for off-label use to a patient is if the peptide itself is already FDA approved for an existing medical condition.
And when doing so, they would hopefully be operating within the bounds of good medicine and using sound clinical judgment.
From a legal standpoint, it may not be a top priority for the FDA to hunt down doctors who are prescribing unapproved peptides to their patients.
But it would not be classified as a legal action, and one would argue this practice continues to happen due to a lack of enforcement.
The word ‘peptide’ isn’t the issue…it’s whether a drug substance is FDA approved or not.
This would therefore mean that SOME of the stuff made by SOME of the compounding pharmacies are not entirely legitimate (i.e. peptides which are dubbed as ‘research chemicals’).
Such is the nature of the game – you have to play within a closed circuit and follow the rules.
As of this writing, there are less than 500 therapeutic peptides that have been officially approved by the FDA.
The Latest FDA Move That May Be A Death Sentence For Therapeutic Peptides

I informed my private circle of close friends about some horrifying news which would affect therapeutic peptides:
“As some insulin, human growth hormone and other products transition on 23 March from new drug applications (NDAs) to biologics license applications (BLAs), the recently passed government spending bill included a further tweak to add new proteins to the transition.
The NDA to BLA change effectively means that any follow-on products for these NDAs will need to win approval as biosimilars after [March 23, 2020]. The transition was created by the Biologics Price Competition and Innovation Act of 2009, which clarified the statutory authority under which certain protein products will be regulated by amending the definition of a “biological product” to include a “protein (except any chemically synthesized polypeptide).”
For a comprehensive list of the drugs the FDA plans to transition towards being classified as BLAs, go here.
You’ll see a LOT of peptides on that list!
Here is how this announcement affects compounding pharmacies:
“What this means is that pharmacies will no longer be able to compound HCG [human chorionic gonadotropin] without a “Biologics License”.
Conventional pharmacies that compound and dispense medications fall under the 503A pharmacies under the FD&C Act. In short, these 503A pharmacies will not be able to acquire a “Biologics License” and therefore they will not be able to compound HCG.”
We’re not just talking about HCG…mendropins, follicle-simulating hormone, hyaluronidase — every peptide you saw on the FDA list can no longer be compounded into medical formulations.
This is much more than a mere formality.
There are a great deal of similarities between NDAs and BLAs:
“The drug is safe and effective for the proposed use and that the benefits outweigh the risks
The labeling is appropriate and contains all necessary information about the drug
Manufacturing methods preserve the drug’s identity, strength, quality, and purity”
However, here is where the difference lies between the two:
“because biological products are processed from living material, BLA content must also demonstrate purity specifically in terms of showing that the final product does not contain extraneous material.
Due to the complexities of manufacturing biological products, a pre-license inspection of the facility is generally required before a BLA is approved. Pre-approval inspections sometimes also take place during an NDA review, but are typically conducted based on risk assessment by the Agency.”
Want to know how expensive an APPLICATION for a BLA is?
As of 2017, the fee is $234,495 ($58,624 if you are a small business).
And a REVIEW of your BLA would set you back another $4569.90.
In total, you’re burning through millions of dollars in program fees, application fees, and the money needed to generate enough clinical data.
Which leads to how FDA generates its operating revenue: 80% from prescription drug program fees and 20% from application fees.
Fortunately, any peptides previously approved as an NDA before March 23, 2020 will eventually be approved as an BLA.
But what about the newer peptides?
What this act does is turn the entire therapeutic peptide industry into one that is too unsustainable for any form of financial success.
And since some compounding pharmacies conduct 50-75% of their business through these peptides, you’re basically putting them out of business.
The only feasible way out would be to have your peptide of choice examined under a study which is monitored and approved by an Institutional Review Board (IRB):
“an IRB is an appropriately constituted group that has been formally designated to review and monitor biomedical research involving human subjects.
In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research.
This group review serves an important role in the protection of the rights and welfare of human research subjects.”
But getting approval from an IRB involves an inhuman amount of complex legal paperwork, not to mention the several thousands of dollars in fees you have to pay PER peptide!
For a single doctor’s office, it’s just not worth the effort, and even the larger companies will not want to touch the IRB application.
Keep in mind that many tip-of-the-spear peptide physicians are not doing this to get rich.
The picture only gets darker from here…
The Biggest Flaw In The FDA Drug Approval Process

So it’s obvious by now that the FDA is the “eye of Sauron” when it comes to determining what does and doesn’t get on the legal drug market.
Where you would be wrong is assuming their entire process is totally legitimate.
The best way to demonstrate this would be through the lab mice being used to conduct animal testing for drugs, which is done long before any human trials are conducted:
I highly recommend you watch the entire video, but here’s a brief summary…
Laboratories have an incentive to breed mice that grow as fast as possible.
More grown mice means more breeding can happen much sooner, which leads to the production of more mice than can be sold off for a hands profit.
Sounds like a perfectly economical thing for a business to pursue, right?
You bet it is — we now know that the United States were not using wild mice for their study, but lab-bred mice which came almost entirely from one source: Jackson Laboratory.
In other words, we are not using mice in their natural habitat, where they are surrounded by hungry predators looking for a good meal.
Here’s where this practice becomes a big deal…
This kind of breeding process favors mice who develop the fastest, and these same mice tend to have abnormally long telomeres.
Telomeres are small DNA sequences located at the ends of your chromosomes and allow your cells to divide in a way where important DNA sequences are retained.
(I’m drastically oversimplifying the science here, but the concept is equally valid)
With each division, the telomeres get shorter, and eventually they become too short to the point where the cells stop dividing and reach a state of senescence.
Studies show that mice with super-long telomeres tend to live much longer, have superior metabolic health and are on average much leaner.
Here’s another important part of this process you need to know about…
The length of these telomeres is maintained through the action of an enzyme called telomerase.
Once telomerase is inactivated, that’s when you get cellular senescence.
Telomerase is present in very high concentrations within cancer cells, which explains why they can divide and replicate themselves so aggressively.
This is also the same reason why these mice, despite living longer, eventually contract cancer and die from it.
This was proven in 2002 by scientist Bret Weinstein when he published the “reverse-capacity hypothesis”:
“…the telomeric differences between humans and laboratory mice have led scientists to underestimate the risks new drugs pose to humans in the form of heart disease, liver dysfunction, and related organ failure”
Here was the conclusion he wrote in the original paper:
“…captive-rodent breeding protocols, designed to increase reproductive output, simultaneously exert strong selection against reproductive senescence and virtually eliminate selection that would otherwise favor tumor suppression.
With their telomeric failsafe effectively disabled, these animals are unreliable models of normal senescence and tumor formation.
Safety tests employing these animals likely overestimate cancer risks and underestimate tissue damage and consequent accelerated senescence.”
These same mice are being liberally used in an endless number of scientific studies, which means that drugs which have no business getting approved receive the green light:
“Longer telomeres delay negative drug side effects. As lab mice are frequently used for drug testing, this could potentially lead to the approval of unsafe drugs.”
“All of the science that’s stacked on these mice that’s contingent on their function relative to their telomeres is all compromised”
So what have in essence are “Wolverine-like” mice that can heal exceptionally well.
As a hypothetical example, if you were to investigate a drug for its potential to damage heart muscle in these mice, the results generated would be abnormal in nature.
These lab mice are NOT representative of what could happen, as they can regenerate heart tissue far better and faster than wild mice.
Does this mean a very large portion of the scientific studies done in the past few decades are invalid and therefore irreproducible?
We don’t know, but some people believe it does:
“We try to control everything we can possibly think of, and as a result we learn absolutely nothing.
Garner argues that research based on mice would be more reliable if it were set up more like experiments in humans — recognizing that variation is inevitable, and designing to embrace it rather than ignore it.
…using animals as models of disease is a big reason that many results in biomedical research aren’t readily reproducible.”
Perhaps this finding invalidates certain types of studies, while others are still legitimate.
But until we can acknowledge this, comprehensively prove it and make changes to address the issue, there is no definitive way to know.
Examples Of Therapeutic Peptides That Are Superior To Conventional Medical Approaches
There are two reasons I can think of for any desire to ban therapeutic peptides from getting in the hands of everyday people.
- Either they are so incredibly dangerous to the point where they can’t be used productively, or
- They are so insanely effective to the point where they can hurt the profits made by existing medical treatments that are inferior in nature.
I’m betting on the latter, and I’ll show you why with some concrete examples.
Sermorelin
Sermorelin used to be one of the most effective peptides used by age management physicians to treat growth hormone deficiency in adults.
Multiple compounding pharmacies were making this peptide and it was very affordable for patients to use.
Sermorelin was thought to be superior to ordinary human growth hormone (HGH) injections that attempted to merely replace a lack of HGH in the body through exogenous administration.
Unlike these injections, Sermorelin acts by helping the body naturally secrete more HGH and release to parts of the body where it is most needed.
This happens because its effect is identical to Growth Hormone-Releasing Hormone, which stimulates the pituitary gland to increase HGH production.
On top of being safer and carrying a far lower risk for side effects, it also provided numerous health benefits (this list is just a very small sample):
“Sermorelin enhances the Pituitary Gland’s natural ability to produce endogenous Human Growth Hormone, leading to a more natural Hormone Profile
Sermorelin optimizes the human body, increasing physical and mental performance
Sermorelin strengthens immune health
Sermorelin boosts the liver’s production of Insulin-Like Growth Factor One
Sermorelin helps patients sleep longer, deeper, and better”
However, its production was sadly discontinued in 2008 “due to difficulties in the manufacturing process of the active ingredient used to produce commercially supplied sermorelin but not due to safety issues.”
And Sermorelin is listed as a banned substance in sports organizations such as WADA and USADA for its anabolic effects, which likely incentivized its discontinuation.
Granted, there are several other HGH-producing peptides that have come into existence and demonstrated favorable results.
But you have to wonder why such an effective biomolecule was suddenly made unavailable1
Cerebrolysin
Cerebrolysin is one of the peptides I plan to feature in my upcoming book about using therapeutic peptides to achieve optimal health.
On top of helping with mild to moderate cognitive impairment, it works phenomenally in helping people recover from traumatic brain injury.
There are HUNDREDS of papers written about Cerebrolysin and its benefits for those people with neurodegenerative disease.
Unfortunately, its newfound classification as a biologic means you won’t be able to get it from any compounding pharmacy, nor will it be available in the US.
Which means you’ll have to use it in other countries if you want to go the legal route:
“Cerebrolysin is currently approved for use in 44 countries as a treatment for dementia and stroke and is in Phase III trials in multiple countries in Europe
…While Cerebrolysin is approved for use in Austria, China, Germany, Russia and South Korea, the FDA has yet to approve it for use in the United States”
If you look at Cerebrolysin’s page on the FDA website, you’ll see that it couldn’t even get approval as an orphan drug!
Tesamorelin
Tesamorelin is a different beast as it is technically FDA-approved.
Specifically, its brand name is Egrifta and it was approved in 2010 for HIV-positive patients with lipodystrophy (i.e. a condition where the body has excessive abdominal fat due to its inability to properly utilize and store fat tissue).
This indication came about because lipodystrophy was one of the major side effects that came with using highly active antiretroviral therapy (HAART) drugs.
But as I wrote in Living A Fully Optimized Life, Tesamorelin is THE BEST peptide for fat loss…especially when combined with a fasting protocol such as Metabolic Blowtorch Diet or Guaranteed Shredded).
It can also lower your blood triglycerides and improve cognition.
Of course, all of these additional uses I mentioned are off-label.
Imagine how many millions of people would benefit from the regular use of Tesamorelin, and yet it is limited to an extremely narrow use.
But think of the health insurance companies – they can cover the drug and make a lot of money doing so.
It’s just the way the system is set up: People in the pharmaceutical industry are there to make more money, pure and simple.
A great way to profit off a peptide without having it interfere with the billion-dollar revenue streams generated by other prescription drugs!
BPC-157
I have written prolifically about BPC-157 in a prior blog post that talked about its amazing healing and acceleration recovery properties.
And it’s important here because I want to expand upon what I talked about with Tesamorelin.
Big Pharma will only push to approve peptides if there is an observed economic demand, and if fulfilling this demand makes financial sense.
Put another way, they WILL NOT bring anything to the market that will negatively impact another revenue stream.
Let’s look at this through two hypothetical yet perfectly realistic examples.
The orthopedics industry amassed a global revenue of $51 billion in 2018, with the top dog being Stryker at $13.6 billion in revenue for the year.
There is an ungodly amount of money to be made from medical devices and surgical equipment such as cervical plates, bone screws, implants, and the list goes on forever.
And what are all these things designed to do?
The EXACT same thing that BPC-157 can treat:
“Orthopedic surgery focuses on the diagnosis, treatment, rehabilitation and prevention of diseases of the bones, joints, ligament, muscles, tendons and nerves”
If BPC-157 ever reached mainstream statis, at least 10-15% of the market would be lost.
This is a massive hit, and the industry wouldn’t like it if some tiny little peptide took their precious billions away from them.
The would either pigeonhole the peptide altogether, or find a way to make money from it without blowing up the products and devices which continue to make them money.
It goes deeper, because even the surgeons stand to benefit.
What medical schools often do is work closely with device manufacturers.
The manufacturers invite doctors for a free demonstration which shows them how to use their devices on patients (using human cadavers as demo objects).
Both sides benefit from this arrangement: Doctors get trained, and the manufacturer gets their products bought and used (along with their name on the building).
What do you think would happen if the surgeons suddenly turned around and said, “Don’t bother with surgery, just use BPC-157 for a few weeks and you’ll be fine”?
I’ll let you answer that question for yourself.
Another area where BPC-157 faces great sabotage is in the area of gastrointestinal (GI) health.
BPC-157 has been repeatedly shown to confer multiple benefits for gut-related conditions such as inflammatory bowel disease and stomach ulcers.
On the other hand, you have the purple pill known as “Nexium” for treating acid reflux, stomach ulcers, and heartburn.
This pharmaceutical wonder brought in $1.48 billion for AstraZeneca in 2019, and made $5.22 billion at its peak in 2007.
As a proton pump inhibitor (PPI), it works by inhibiting the release of hydrochloric acid into the stomach.
But when used at higher dosages for too long, it can lead to some serious problems:
“Proton Pump Inhibitors (PPIs) including Nexium and its “parent” drug, Prilosec, have also been shown to increase the risk of heart attack, bone fracture and hypomagnesemia or low magnesium levels.
PPIs are also suspected of causing other serious conditions such as acute kidney injury. birth defects, and acquired infections.
Multiple lawsuits have been filed against the manufacturer of Nexium and many more are expected.”
You have to keep on taking the drug to TREAT the problem instead of FIXING it.
Why on earth would a pharmaceutical company want to kill off such a profitable drug with a peptide that is much more effective, actually addresses the root cause and has virtually no side effects?
Peptides Will Never Compete With Medically Profitable Revenue Streams
Therapeutic peptides, whether natural or synthetic, are backed by an ever-increasing body of medical research which only confirms how effective and safe they are.
People have a better quality of life and have better long-term results when using them.
Plus, many health optimization doctors have successfully used them for decades with their patients.
They are simultaneously a dream come true for mankind and the worst possible nightmare for any pharmaceutical industry.
With everything I’ve told you, let’s recap what companies will do to keep peptides away from public recognition.
You take a therapeutic peptide with widespread potential for treating multiple conditions and have it approved for targeting a very rare and/or undiscovered condition.
Once you get the stamp of approval from the FDA, you charge a shitload of money for it.
You add an additional source of income without jeopardizing the revenue streams you have already built.
Should you catch someone frequently using the peptide off-label (i.e. what it was really meant for), you can have your attorneys go after them and make their lives as miserable as possible.
But how do you track when and why your drug is being used?
Through something called a prescription drug monitoring program (PDMP), otherwise known as a prescription monitoring program (PMP).
Here’s a brief description of how these programs work:
“…[they are] state-run programs which collect and distribute data about the prescription and dispensation of federally controlled substances and, as the individual states deem appropriate, other potentially addictive or abusable prescription drugs.”
A PMP allows you to track all of the drugs being used, who is prescribing them, the person they’re prescribed to, the indication they’re used for, and everything else you could want to know about how your drug is being distributed.
Realistically, the alarms won’t sound if you see just a handful of doctors using a competitive peptide off-label.
But beyond that, it’s time to have a “quiet” conversation with the guilty doctors.
You legally intimidate them, pressuring them to stop using the peptide for off-label purposes or else the peptide will no longer be supplied to their pharmacy.
If you don’t want to be the bully yourself, you can call the FDA!
You just ring them up and say “We saw these doctors using the peptide for conditions not approved for with our drug, and based on our data we do not approve of this.”
The FDA calls those doctors and asks them why the drug is being prescribed for an ailment which is not recommended by the manufacturer.
I could go on, but there are many different ways to exert a lot of pressure on doctors who dare to deviate from the sick care model of healthcare.
This is not the “grand conspiracy theory” many health researchers believe it is, but pure economics and psychology in action.
It’s a natural feedback loop in which people will do whatever it takes to make as much money as possible in the free market society, even if that means suppressing a superior solution.
Most of humanity is sadly asleep and low conscious — they are not going to push back and fight against a system they are unconsciously dependent on.
How The FDA’s Efforts Will Create A Black Market For Therapeutic Peptides
So with therapeutic peptides out of the picture, where else are people going to get them?
As with any drug that becomes illegal, you are going to see a grey market – and possibly a black market – created for these peptides.
“Grey goods are legitimate goods that come from the correct manufacturer, but they are sold through unauthorized channels. While these are not illegal goods, it is important to be wary of them. Sellers who use the grey market will usually sell products that have been improperly discarded due to damage or product recall.”
“Black market goods are items that are illegal to manufacture or sell. Some black market goods are counterfeit, and some are genuine products that are simply illegal to own or distribute. These are usually a part of a separate, more obscure market than grey goods.”
Without any form of regulation, malicious sellers can get away with selling over-priced peptides that are impure and contaminated:
“As a result, the final price on gray market–traded drugs may be as much as hundreds of times higher than the price that the manufacturer originally received for the product.
Also, as the drugs bounce along the extended supply chain, they may be improperly repackaged, re-labeled, and possibly stored under unsuitable conditions, as well as replaced by counterfeits, compromising their integrity and safety.”
Combine this with the lack of knowledge from the consumer and you have a recipe for disaster.
Think about it: The average person does not know how to properly inject themselves or handle peptides properly!
My prediction is not far removed from reality, as this is already happening with prescription drugs today:
“Due to drug shortages, 52 percent of hospital supply chain employees and pharmacists have been forced to buy drugs from the “gray market,” according to ISMP research.
The gray market consists of drug suppliers that are somehow able to obtain supplies of scarce drugs and sell them at exorbitant costs, sometimes with mark-ups as high as 650 percent, according to previous studies.”
This is the reality we will soon face unless massive and immediate action is taken.
But there’s just one more hurdle we have to deal with…
We Have VERY Few Physicians Who Can Competently Prescribe Peptides
When you do a head count of all the physicians who are certified and well-trained to safely administer therapeutic peptides, you’re talking about a couple hundred at best.
This is an extremely niche area of medicine which requires extensive training and knowledge you won’t get in 4 years of medical school.
And there are very few highly-accredited teaching bodies in existence:
- The American Academy of Anti-Aging Medicine
- Peptide Practices of America
- Peptideology (courtesy of Dr. Heather Smith-Fernandez)
- International Peptide Society
The few peptide physicians in existence have had to put a significant amount of time and money towards obtaining their fellowship training in peptide therapy.
They have the first-hand experience, the connections to legitimate compounding pharmacies, and the testimonies from thousands of patients who can attest to both their doctors and the life-changing benefits of therapeutic peptides.
But without the FDA allowing the use these peptides, these doctors will not have the tools necessary to properly care for their patients.
What You Can Do To Save The Future Of Therapeutic Peptides

We need to collectively come together and do everything we can to stop the FDA from suppressing the use of therapeutic peptides altogether.
So here are some action steps you can take immediately to do your part and have your voice heard.
First, go to SavePeptides.org and tell the world how therapeutic peptides have changed your life for the better.
Every single submission matters, so spare no details in telling the world why we MUST keep these miraculous compounds available for human use.
Staying on the sidelines with off-label use is no longer acceptable!
Second, you can contact the FDA and explain why banning therapeutic peptides will negatively impact the lives of tens of thousands of people.
This website provides you with a good starting template if you need assistance with writing a thoughtful reply.
Third, you always have the option of getting your peptide of choice approved through the IRB process.
This is not a perfect approach and it is extremely expensive and time-consuming.
But if this actually works in your favor, you can have a reputable compounding pharmacy make the peptide for your intended purpose.
Fourth, make sure you support the organizations that work tirelessly to keep the clinical use of peptides alive and running.
All the organizations I listed in the previous section are doing tremendous work in advancing the scientific research of peptides, both old and new.
Just because peptides are currently unapproved by the FDA or used off-label DOES NOT mean that they are inherently unsafe!
Fifth, and finally, share the videos and podcasts I have released on the subject of therapeutic peptides.
The more people know about what therapeutic peptides can do, the better society will be.
This Podcast is the BEST EVER DONE on understanding how to use therapeutic peptides for nearly every conceivable clinical indication.
(CLICK HERE to get the full transcript of this podcast)
And as always…
If you want access to the world’s best health optimization intel before anybody else finds out about it, subscribe to my email list!



